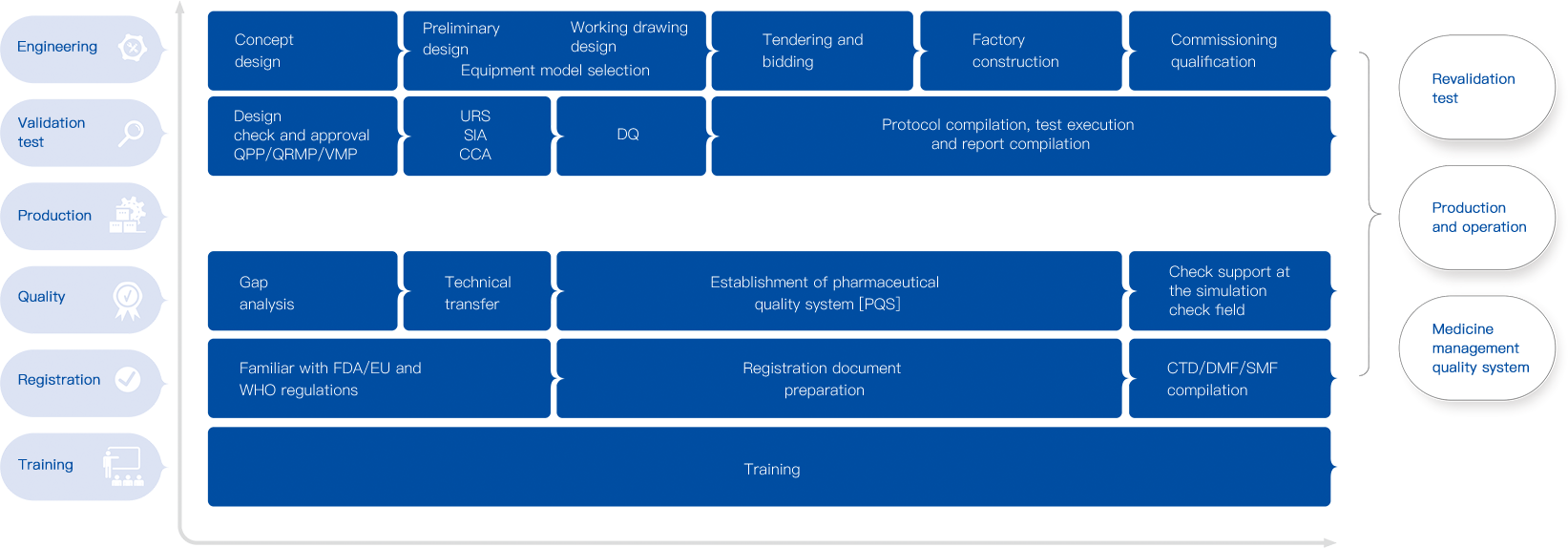
Juchuang has a professional team and various senior consultants for providing GMP compliance and quality management consultation services.
We have provided GMP services for several hundreds of pharmaceutical enterprises. Juchuang is able to provide professional and comprehensive GMP compliance validation consultation services that conform to the requirements of FDA / EU / CFDA and WHO.
- • Ensuring that the materials during production are those passed by the quality department
- • Ensuring that the materials during production have the correct commodity name, specification, production lot and quantity
- • Ensuring that the produced intermediate is identified by the barcode to avoid mixed use of materials
- • Ensuring that the room and equipment in use are clean
- • Ensuring that the container that contains the intermediate is clean
- • Ensuring that the materials are fed according to the lot No. during feeding
- • Ensuring the completeness, effectiveness and accurateness of records

 中文
中文  ENGlish
ENGlish